Gabapentin,API,Active Pharmaceutical Ingredient - Gabapentin,API
Gabapentin,API,Active Pharmaceutical Ingredient For Sale, Most Competitive Price, Fast Delivery, Custom Service, Wholesale Gabapentin,API,Active Pharmaceutical Ingredient, Made in China, High Quality Products!, China medical intermediates, pharmaceutical intermediates Supplier, Manufacturer.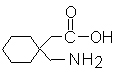
Gabapentin,API(Active Pharmaceutical Ingredient)
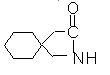
Gabapentin is described as 1-(aminomethyl) cyclohexaneacetic acid with a molecular formula of C9H17NO2 and a molecular weight of 171.24.
CAS NO: 60142-96-3
Assay: ≥99.0%.
According to USP standard.
Description:
Gabapentin is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely soluble in water and both basic and acidic aqueous solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is ?
We can offer high quality Active Pharmaceutical Ingredient with competitive price.Our email address is rypharma at yahoo dot com
Xiamen Xiangluda Medical Instrument Co.,LTD.
Address: Lubin Building,No.69,Dongdu Road, Xiamen, Fujian, China, 361012
Tel: 0086-592-3868333
Xiamen Xiangluda Medical Instrument Co.,LTD. has been engaged in distribution and management of API(Active Pharmaceutical Ingredient) and pharmaceutical intermediates with many years’ experiences.
We have developed lots of new products and have good relationships with many manufacturers. We export a wide range of pharmaceutical products with high quality and competitive price.
Here are our new products:
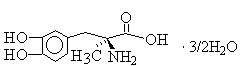
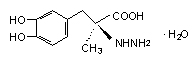
API(Active Pharmaceutical Ingredient): Etodolac; Gabapentin; Carbidopa; Methyldopa ;etc.
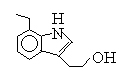
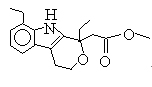
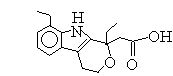
Medical and Pharmaceutical Intermediate: 7-Ethyl tryptophol; Etodolac methyl ester; 3,3-Pentamethylene-4-Butyrolactam;etc.
We are working hard on applying for the FDA and COS authentification of all the above products.
If you have interests in any of the items, please don’t hesitate to send us your inquiry against which we will offer our best quotation.
It will be a great pleasure if we can be of service of you and your esteemed company.